
You can reduce transfer time by arranging your workspace for shorter distances and clear paths. Fast and careful transfer lowers contamination risk and keeps products sterile. Quick transfer time protects product quality and helps your team work efficiently. Use strong environmental controls and train your staff well to support safe and fast transfer every day.
Transfer Time Factors

Layout and Distance
You can improve transfer time by designing your workspace with efficiency in mind. Shorter distances between the Primary Engineering Control (PEC) and the box area help you move materials quickly. A direct path reduces the risk of contamination and supports better workflow performance. When you plan the layout, you should avoid obstacles and keep the route clear. This approach helps you maintain product sterility and supports optimization of your daily operations.
Tip: Place the PEC and box area close together, but ensure enough space for safe movement and proper airflow. This balance helps you achieve both speed and safety.
Material Handling Methods
The way you handle materials has a direct impact on transfer time and contamination risk. You can use several proven methods to optimize performance and reduce challenges:
Use dedicated cleanroom carts that you disinfect before each use.
Manage waste with separate pathways to prevent cross-contamination.
Employ barrier systems, such as pass-through chambers with HEPA filtration and UV-C lighting, for decontamination.
Utilize airlocks with pressure differentials to keep contaminated air out.
Apply staged transfer methods, moving materials through cleaner zones step by step.
Follow minimal touch principles to limit human contact.
Package materials with cleanroom-compatible supplies.
Use decontamination protocols like chemical sterilization or vaporized hydrogen peroxide, depending on material needs.
Material Handling Method | Purpose/Function | Impact on Transfer Time and Contamination Risk |
|---|---|---|
Gowning Procedures | Minimize personal contamination | Reduces particle introduction, lowering contamination risk |
Material Preparation | Proper cleaning and packaging | Facilitates smoother and faster transfers, reducing transfer time |
Automated Transfer Systems | Use of robotic arms, conveyor belts, pneumatic tubes | Minimizes human intervention, reducing contamination risk and speeding up transfers |
Dedicated Cleanroom Carts | Use carts specific to cleanroom environments | Prevents cross-contamination, maintains cleanliness during transfer |
Disinfecting Carts Before Use | Cleaning carts, especially wheels | Reduces contamination risk from equipment |
Waste Management via Separate Pathways | Separate routes for waste removal | Prevents cross-contamination between waste and clean materials |
Barrier Systems (Pass-Through Chambers with HEPA and UV-C) | Physical separation and decontamination | Controls particle movement, reduces contamination risk |
Airlocks with Pressure Differentials | Maintain positive pressure in cleanrooms | Prevents inflow of contaminated air, maintaining clean environment |
Unidirectional Airflow | Controls air movement | Minimizes particle spread during transfer |
Staged Transfer Approach | Moving materials through progressively cleaner zones | Gradually reduces contamination risk |
Minimal Touch Principle | Limiting human contact with materials | Decreases bioburden and contamination risk |
Proper Packaging | Using cleanroom-compatible packaging | Maintains material cleanliness during transfer |
Continuous Training and Risk-Based Protocols | Educating personnel and tailoring procedures | Improves compliance, reduces errors, balances safety and efficiency |
Real-Time Monitoring and RFID Tracking | Environmental and material tracking | Enables rapid response to contamination risks and improves operational efficiency |
Well-implemented cleanroom protocols can reduce contamination incidents by up to 80% and improve operational efficiency by standardizing procedures. When you integrate IoT-enabled sensors and AI-driven analytics into your transfer systems, you can see a 40% reduction in contamination incidents and a 25% increase in operational efficiency. Organizations that focus on risk-based protocols and continuous training report a 50% reduction in contamination incidents and a 35% improvement in cleanroom efficiency.
Personnel Training
Your team’s training and support play a critical role in reducing transfer errors and contamination. Frequent and high-quality training ensures that everyone understands the latest best practices and follows them every time. You should provide regular updates and hands-on sessions to reinforce proper techniques. This approach helps you address challenges and maintain high performance.
An empirical study in forensic science showed that teams with more frequent and thorough training had fewer contamination incidents. As DNA profiling systems become more sensitive, the need for upgraded procedures and ongoing training grows. You can reduce transfer errors and contamination by investing in continuous training and support for your staff.
Note: Make training and support a regular part of your workflow. This habit helps you adapt to new challenges and maintain high standards.
Environmental Controls
Environmental controls are essential for maintaining sterility during PEC to box transfers. You must manage airflow, pressure differentials, temperature, and humidity to reduce contamination risks. Here are some key factors to consider:
Maintain ISO classifications with cleaner air moving toward the PEC, which should operate at ISO Class 5.
Buffer rooms should meet ISO Class 7 standards with at least 30 air changes per hour.
Keep positive pressure differentials of at least 0.020-inch water column between adjacent areas.
Supply air through HEPA filters, with at least 15 air changes per hour from the HVAC system.
Use fixed walls and doors to separate rooms; avoid makeshift barriers.
Monitor pressure differentials continuously to ensure positive pressure.
Control temperature (20°C or cooler) and relative humidity (60% or lower) to limit microbial growth.
Manage personnel and material flow to minimize contamination during transfer.
Quantitative studies show that longer contact times between surfaces and materials increase contamination rates. You can reduce these risks by minimizing contact time and maintaining strict environmental controls. Environmental cleaning in healthcare settings also reduces bioburden and the spread of resistant organisms, supporting better performance and optimization.
Tip: Regularly review your environmental controls and make adjustments as needed. This practice supports ongoing optimization and helps you meet new challenges as they arise.
Effective communication between teams, strong training and support, and a focus on environmental controls all contribute to better transfer time, improved performance, and successful ehr transition. By addressing these factors, you can overcome challenges and achieve a smooth workflow during every ehr transition. Remember, ongoing training and support, clear communication, and a commitment to optimization are key to maintaining high performance in your new ehr system.
Best Practices
Streamlined Workflows
You can achieve better performance by streamlining your workflow. Start by mapping each step from the PEC to the box area. Remove unnecessary actions and focus on value-added tasks. Lean principles help you identify bottlenecks and reduce errors. When you apply these methods, you see faster transfer time and fewer mistakes during ehr transition.
Studies show that 26% of lean implementations lead to faster medication turnaround.
15% of cases report improved process efficiency in pharmacy workflows.
11% of projects find and fix bottlenecks, cutting out wasted steps.
9% of organizations see fewer medication errors, boosting safety.
8% report higher staff and patient satisfaction after lean changes.
3% improve prescription checking and intervention success rates.
Many also see better inventory management and more consistent workflow.
You can see these improvements in real-world settings:
Outcome Metric | Improvement Observed | Context / Example |
|---|---|---|
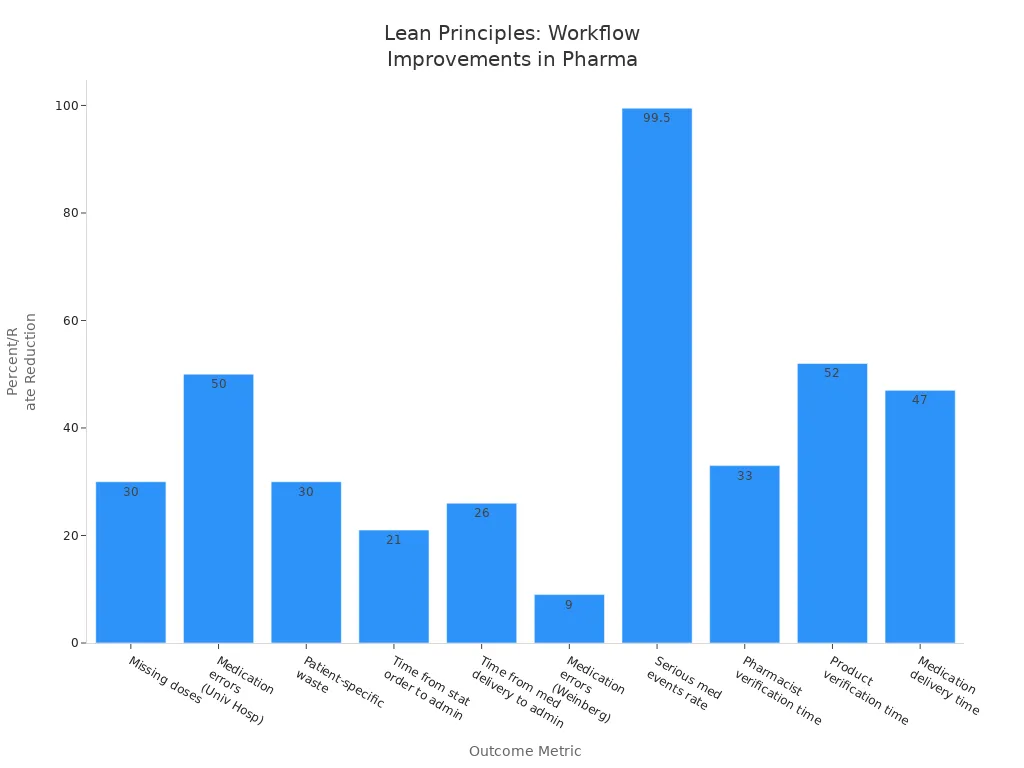
Missing doses | Decreased by 30% | Sterile products and inventory area in a university hospital |
Medication errors | Reduced by 50% | Same university hospital setting |
Patient-specific waste | Reduced by 30% | Same university hospital setting |
Time from stat order entry to administration | Reduced by 21% | US Department of Veterans Affairs hospital using A3 thinking |
Time from medication delivery to administration | Reduced by 26% | Same VA hospital |
Medication errors | Decreased from 50% to 41% | Weinberg Pharmacy, Sidney Kimmel Cancer Center |
Serious medication events rate | Reduced from 1.3 to 0.007 per 10,000 orders | Community hospital in Ontario implementing multiple lean actions |
Pharmacist verification time | Reduced by 33% | Smilow Cancer Hospital quality improvement project |
Product verification time | Reduced by 52% | Smilow Cancer Hospital quality improvement project |
Medication delivery time | Reduced by 47% | Smilow Cancer Hospital quality improvement project |
A study in China found that after lean management, prescription errors dropped from 2,978 to 219. The error rate fell from 3.46‰ to 0.27‰. Patient satisfaction rose from 52.94% to 72.97%. You can reach similar results by standardizing your process and using root cause analysis. These steps support workflow optimization and continuous improvement during ehr transition.
Tip: Use regular monitoring and feedback to keep your workflow efficient. Good communication between teams helps you spot problems early and make quick improvements.
Automation and Mechanization
You can boost performance by adding automation and mechanization to your workflow. Automated transfer systems, such as robotic arms or conveyor belts, move materials quickly and safely. These systems reduce human error and keep your process consistent during ehr transition. Pneumatic tubes and RFID tracking also help you monitor materials in real time.
Automation supports optimization by:
Reducing manual handling, which lowers contamination risk.
Speeding up transfer steps, making your workflow more predictable.
Allowing you to track every item, so you can respond fast if issues arise.
Freeing up staff for higher-level tasks, which improves overall performance.
When you use automation, you also improve communication between teams. Automated alerts and tracking systems keep everyone informed. This approach helps you maintain high standards during your new ehr system rollout.
Note: Always provide training and support for staff when you introduce new automated systems. This step ensures everyone uses the technology correctly and safely.
Aseptic Techniques
You must use strong aseptic techniques to protect product sterility during transfer. These methods keep your workflow safe and efficient, especially during ehr transition. The best-documented aseptic techniques include:
Working inside certified laminar flow hoods or cleanrooms with HEPA filters.
Running the hood for at least 30 minutes before use.
Wearing sterile gloves, lint-free clothing, and masks.
Scrubbing hands and arms with antibacterial agents.
Cleaning surfaces with 70% isopropyl alcohol, moving top to bottom and back to front.
Assembling supplies inside the hood and checking for damage or expiration.
Swabbing all surfaces with alcohol or betadine before entry.
Minimizing hand movements and keeping hands in the proper position relative to airflow.
Handling needles and syringes carefully to avoid touching sterile surfaces.
Working at least 6 inches inside the hood edge and not removing hands until finished.
Inspecting formulations before removal and disposing of sharps properly.
You should also use sterile barriers, such as gloves, gowns, and drapes, to prevent contamination. Limit the number of people in the room and keep doors closed during procedures. Always follow strict contact guidelines so sterile items only touch other sterile items. These steps help you maintain performance and reduce contamination during ehr transition.
Alert: Regular training and support are essential. Make sure your team practices aseptic techniques often and receives updates on best practices.
Validated Protocols
Validated protocols give you a reliable way to maintain sterility and consistent transfer times. These protocols set clear conditions and limits for each step in your workflow. They include detailed sampling plans and acceptance criteria, which give you confidence in your product quality during ehr transition.
Validated protocols help you by:
Setting strict parameters for temperature, time, and pressure.
Using microbiological challenge testing to prove sterilization works.
Requiring environmental monitoring for air, surfaces, temperature, and humidity.
Demanding ongoing personnel training and competency checks.
Ensuring equipment is qualified and calibrated regularly.
Calling for continuous real-time monitoring and documentation.
Requiring periodic reviews and updates for continuous improvement.
When you follow validated protocols, you control bioburden, endotoxins, and particles. You also limit environmental exposure, which keeps your process safe during ehr transition. This approach supports optimization and high performance in your new ehr system.
Tip: Keep communication open between teams. Share protocol updates and feedback often to support continuous improvement and maintain high standards.
Common Pitfalls
Network or Process Bottlenecks
You may face many challenges when you move products from the PEC to the box area during an ehr transition. Network or process bottlenecks often slow down your workflow and put product quality at risk. These bottlenecks can appear in several ways:
Difficulties in transferring data between different entities and ehr systems.
Communication barriers that make it hard to report to stakeholders.
Limited resources, such as workforce shortages, which affect data collection and measurement.
Disruptions to normal workflow caused by the complexity of ehr interventions.
When you do not address these challenges, you see longer transfer times and lower product quality. For example, in biomanufacturing, a bottleneck in the first stage can slow down the whole process, leaving equipment idle and reducing throughput. Bottlenecks also cause delayed deliverables, inventory pile-ups, and overworked staff. These issues lead to low-quality output and more errors. By identifying and fixing bottlenecks, you can improve operational efficiency by up to 25% and reduce labor costs by 10-15%. Addressing these challenges and mitigation strategies can lead to a 57% reduction in delivery time and a 99.8% improvement in system reliability during ehr transitions.
Tip: Use metrics like throughput rate and cycle time to spot bottlenecks early. Quick action helps you keep your ehr workflow smooth and efficient.
Inadequate Training
Inadequate training creates serious challenges for your team during ehr transitions. When staff do not receive enough training, they make more mistakes and increase contamination risk. You may notice more errors, slower transfer times, and confusion about new ehr procedures. These challenges can lead to poor product quality and lower team morale.
You should provide regular, hands-on training to help your team master new ehr workflows. Frequent updates and practice sessions keep everyone confident and ready to handle challenges. Well-trained staff can spot problems early and respond quickly, which protects product sterility and keeps your workflow on track.
Alert: Make training a regular part of your ehr process. Ongoing support helps your team adapt to new challenges and keeps performance high.
Ignoring Environmental Controls
Ignoring environmental controls during ehr transitions can lead to major challenges and contamination events. Lapses in plant design, poor sanitation, and weak employee hygiene have caused outbreaks, such as listeriosis linked to cheese production. In healthcare, poor cleaning of medical devices has led to many preventable outbreaks. Pathogens can spread through equipment, air, liquids, or even an operator’s hands.
You must monitor and maintain environmental controls at every step of the ehr process. Regular inspections and environmental monitoring help you find and fix problems before they cause harm. When you ignore these controls, you risk product contamination and patient safety.
Note: Always check your environmental controls during ehr transitions. Strong controls protect your products and help you overcome challenges.
Workflow Example
Preparation
You set the stage for a successful transfer by preparing your workspace and team. Start by reviewing your ehr protocols and ensuring all materials are ready. Assign roles based on each team member’s skill and availability. This step reduces idle time and prevents bottlenecks. Clean and disinfect all surfaces and equipment. Check that your environmental controls meet the required standards. Use a checklist to confirm that you have all supplies and documentation in place. Preparation helps you avoid delays and supports a smooth ehr workflow.
Tip: Early collaboration between your development and manufacturing teams improves process understanding and predictability during ehr transitions.
Execution
You carry out the transfer by following a clear, step-by-step process. Assign tasks to team members based on their strengths and the equipment available. Prioritize critical steps that affect your ehr timeline. Use lean principles like just-in-time movement and waste reduction to keep the process efficient. Monitor your progress in real time with tools such as Manufacturing Execution Systems (MES). MES gives you full traceability, reduces human error, and ensures you meet quality standards. You can quickly address any issues that arise and keep your ehr transfer on track.
Optimize resource allocation to reduce downtime.
Prioritize tasks that impact the ehr production timeline.
Consider equipment maintenance and supplier schedules.
Use MES for real-time monitoring and control.
Foster continuous improvement by reviewing performance after each transfer.
Note: Technology like MES helps you make faster decisions and maintain compliance during ehr transitions.
Verification
You verify the success of your transfer by checking key metrics and documenting results. Set up dashboards to track data success rates, error rates, and support tickets. Aim for a data success rate above 99.9% for your ehr transfers. Use automated alerts to detect issues within minutes. Review your results daily, weekly, and monthly to ensure ongoing quality.
Step | Action | Frequency |
|---|---|---|
Set Up Data Sources | Connect databases, APIs, spreadsheets | Once at setup |
Define Data Points | Map required metrics to sources | Monthly review |
Check Data Quality | Verify accuracy and completeness | Weekly |
Set Up Alerts | Configure notifications for issues | Update quarterly |
Report Type | Content | Update Schedule |
|---|---|---|
Daily Brief | Error rates, uptime, usage | Every 24 hours |
Weekly Summary | User adoption, data flow | Every Monday |
Monthly Review | Revenue impact, partner growth | First of month |
Quarterly Analysis | Long-term trends, ROI | Every 3 months |
Alert: Regular verification and documentation help you maintain high standards and support continuous improvement in your ehr workflow.
You can achieve faster ehr transfer times and maintain sterility by streamlining workflows, using validated protocols, and applying strong environmental controls. Regular feedback and improvement, along with ongoing training, help your team adapt to new ehr challenges. Studies show that clear communication tools and community engagement reduce contamination and speed up ehr processes. Continuous improvement relies on process monitoring, staff involvement, and open dialogue. Review your ehr protocols often and encourage your team to share ideas for improvement.
FAQ
What is the most important step to reduce ehr transfer time?
You should focus on streamlining your workflow. Map each step in your ehr process. Remove unnecessary actions. This approach helps you move materials faster and keeps your products safe.
How does staff training impact ehr transfer efficiency?
Regular training ensures your team understands ehr protocols. Well-trained staff make fewer mistakes. You see faster transfers and lower contamination risk. Training also helps your team adapt to changes in ehr procedures.
Why do environmental controls matter in the ehr workflow?
Environmental controls keep your ehr process sterile. You must monitor airflow, pressure, and temperature. These controls prevent contamination and protect your products during every ehr transfer.
Can automation improve ehr transfer times?
Yes. Automation speeds up your ehr workflow. Robotic systems and tracking tools move materials quickly. You reduce human error and keep your process consistent.
How often should you review ehr transfer protocols?
You should review ehr protocols regularly. Frequent reviews help you find problems early. Update your procedures to match best practices. This habit keeps your ehr workflow efficient and safe.
See Also
Why WarpDriven ERP Excels In Smart Enterprise Management
Ways Top Brands Grow Using WarpDriven Distribution Solutions
The Role Of Distribution Management In Global Business Success